AFROTROPICAL & INDO-ORIENTAL REGIONS
Etymology: not stated [gnat-face (L); gnat resembling]
Early records of An. culicifacies were called An. listoni Giles—now in synonymy with An. culicifacies, along with indica Theobald, punjabensis James and adenensis Christophers. Five distinct species-like lineages of An. culicifacies have been recognized on the basis of cytology, referred to as species A, B, C, D and E. Species A, B, C and D are reported from India, and B and E from Sri Lanka; the taxonomic status of other geographical populations remain undetermined. Biological differences are apparent in the behavior, vectorial capacity, distribution and seasonal prevalence of the putative species, but it remains uncertain whether any of these taxa conform to any available synonyms.
Type locality: Hoshangabad [Central Provinces], India
Type depository: Natural History Museum, London, England, United Kingdom (NHMUK)
DIAGNOSTIC CHARACTERS (Click photos to view; mouse over and click large photo to zoom in.)
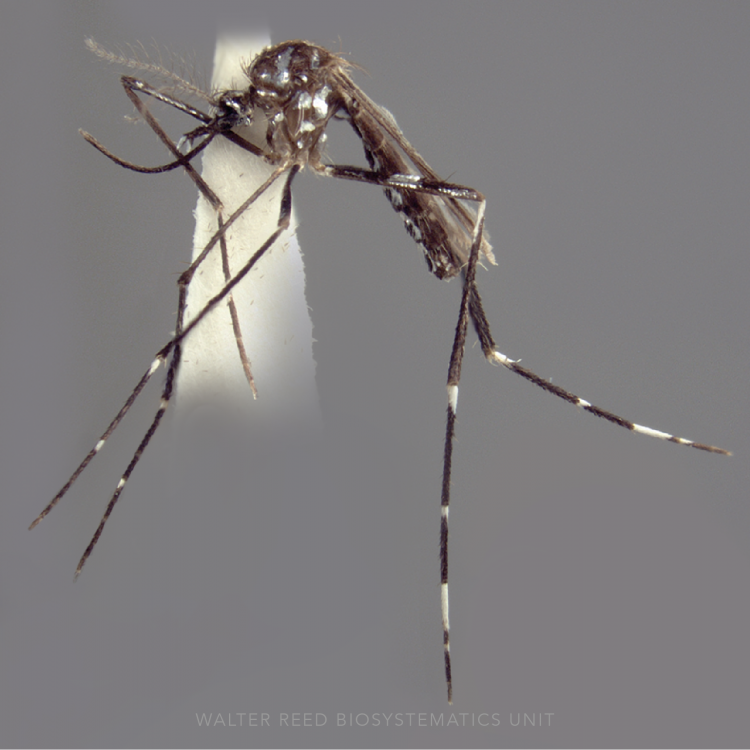
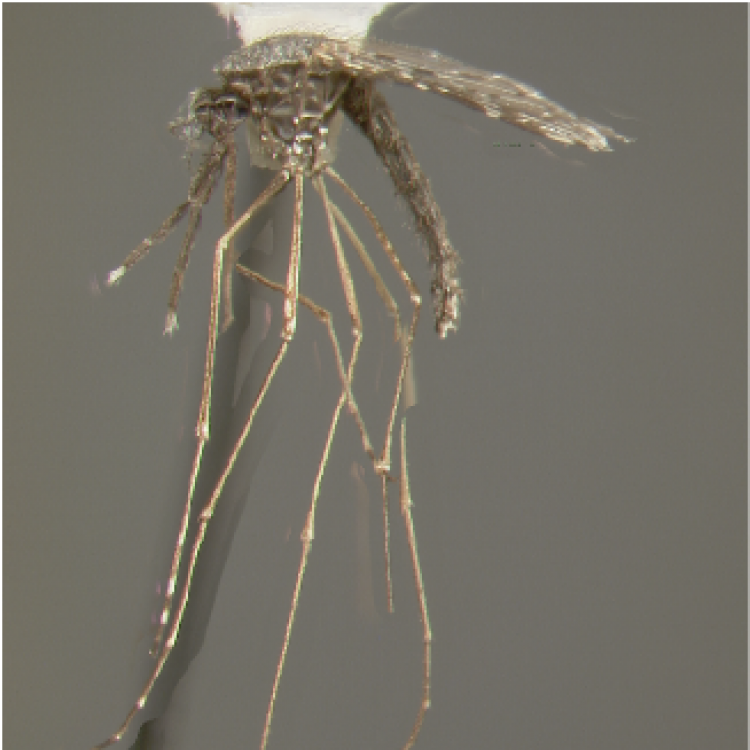
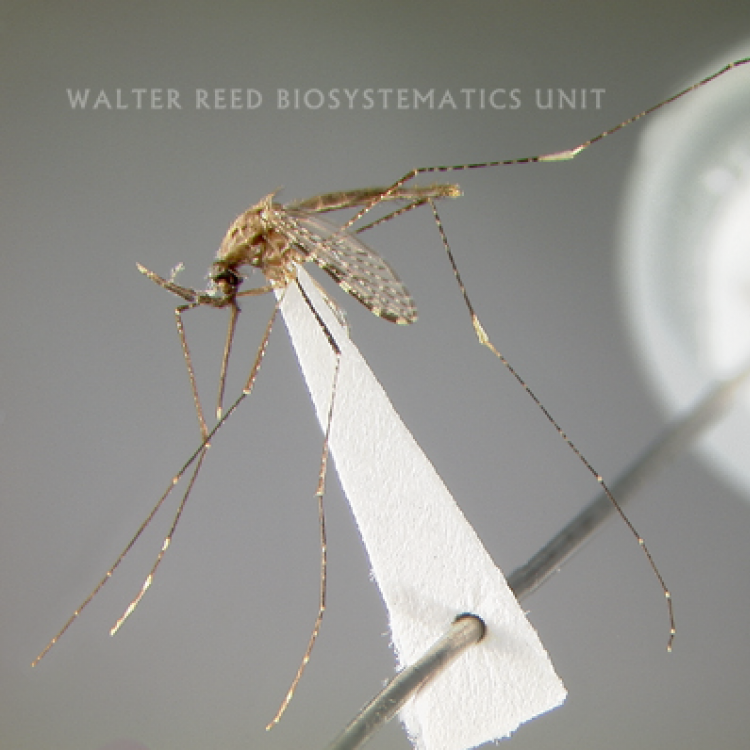
ADULT (illustrated): Head: Palpus with 3 pale bands, apical pale band much shorter than preapical dark band. Thorax: Center of scutum lacking scales or with very slender white scales; antepronotal scales absent. Wing: Vein R1 without a pale interruption at preapical dark spot; vein R4+5 dark, except at base; remigium dark-scaled. Legs: All dark or with some pale patches or pale bands on apices of tarsomeres.
LARVA (not illustrated): Head: Seta 1-A much reduced; seta 2-C widely separated; setae 5–7-C long, branched. Terminal segments: Segments IV-VII with small tergal plate, not enclosing median accessory tergal plate.
TAXONOMIC KEYS
Nguyen Thuong Hien 1968
Darsie & Pradhan 1990
Rattanarithikul et al. 2006b
Rattanarithikul & Harrison 1973
Becker et al. 2010
![]()
WRBU – Anopheles - Myzomyia Series - Oriental Region - Larva
![]()
WRBU - Anopheles - Western Palearctic Region - Adult
![]()
WRBU - Genera - Global - Adult
![]()
WRBU - Genera - Global - Larva
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Larva
![]()
WRBU - Anopheles Subgenera and Series - Oriental - Adult
![]()
WRBU - Anopheles Subgenera and Series - Oriental - Larva
Exemplar DNA sequences
An. culicifacies A: rDNA ITS2: AF402297, AJ534246, EF462897
An. culicifacies B: rDNA ITS2: AY167747, EF462896, AJ534247
An. culicifacies C: rDNA ITS2: AJ534643
An. culicifacies D: rDNA ITS2: AJ534644
An. culicifacies E: rDNA ITS2: AY168883
BIONOMICS
Immatures
Typical immature habitats for An. culicifacies s.l. include the grassy edges of clean, sunlit slow-moving streams, drainage ditches, fallow rice fields, rain pools, canals, pools in river beds, shallow tanks, pits and wells. Immatures are occasionally found in brackish water sites. Immatures have been collected at a variety of elevations, depending on geography—between 35–960m in Thailand, only ever above 960m in Vietnam, and as high as 2286 m in Pakistan, and up to 6,500 ft in the Himalayas. In northern India, or at high elevations, the species overwinter as larvae.
Adults
Anopheles culicifacies s.l. are generally associated with riverine floodplains. Anopheles culicifacies s.l. are predominantly anthropophilic, although some populations are reportedly zoophilic, preferring instead to feed on cattle and birds. Species A,C, and D are able to effectively transmit malaria, however they preferentially feed on domestic animals. Species E is a highly competent malaria vector in southern India and Sri Lanka. Species B is zoophilic and is not implicated in human pathogen transmission. Females rest in huge numbers in cool, dark houses or cattle sheds during the day to mature their eggs.
DISTRIBUTION NOTES
Afghanistan, Bahrain, Bangladesh, Bhutan, Cambodia, Cyprus, Eritrea, Ethiopia, India, Iran, Iraq, Israel, Kazakhstan, Laos, Malaysia, Myanmar, Nepal, Oman, Pakistan, People's Republic of China, Saudi Arabia, Somalia, Sri Lanka, Thailand, Vietnam, Yemen (Socotra).

WRBU VECTOR HAZARD REPORTS
View other WRBU Vector Hazard Reports
Available GIS Models
An_culicifacies_s.l._Foley_1 Asia
IMPORTANT REFERENCES (full citations below)
Giles 1901b: 197 (M, F)
Christophers 1933: 197 (M*, F*, P, L*, E; taxonomy)
Evans 1938: 172 (M*, F*, P, L, E; as adenensis)
Ross & Roberts 1943b: 13 (M*, F*, L*; taxonomy, distribution, bionomics)
De Meillon 1947b: 99 (M*, F*, P*, L*; as adenensis)
Horsfall 1955: 199 (review)
Viswanathan, Rao & Halgeri 1955: 371 (bionomics)
Gillies & De Meillon 1968: 104 (M*, F*, P, L*; taxonomy; as adenensis)
Nguyen Thuong Hien 1968 (F*, L*; keys, taxonomy, bionomics, Vietnam)
Beidas & Gillies 1980: 172 (E*; as adenensis)
Harrison 1980: 55 (M*, F*, P*, L*; lectotype designation)
Ahmed 1987 (distribution; Bangladesh)
Das 1990 (F*) (as species A & B)
Darsie & Pradhan 1990 (F, L; taxonomy, keys, bionomics, distribution; Nepal)
Baimai et al. 1996 (sensu lato; chomosomes)
Kar et al. 1999 (sensu lato; taxonomy)
Rattanarithikul et al. 2006b (F*, L*; bionomics, distribution, keys)
Manonmani et al. 2007 (sensu lato; molecular taxonomy)
Rattanarithikul & Harrison 1973 (L*; key, Thailand)
Becker et al. 2010: 341 (F*, L*; key, taxonomy, distribution, bionomics)
Sinka et al. 2011: 89 (Culicifacies Complex; bionomics review, distribution, niche model)
Dev & Sharma 2013 (taxonomy, bionomics; Culicifacies Complex.)
Sharma et al. 2014 (sensu lato; taxonomy, distribution)
Kyalo et al. 2017 (distribution; sub-Saharan Africa)
Kyalo et al. 2017 (distribution; sub-Saharan Africa; as adensis)
Namgay et al. 2018 (distribution, bionomics; Bhutan)
CURRENT SYNONYMS
syn. indica Theobald
1901a: 183 (F). Type locality: Madras, India (NHMUK).
syn. listoni Giles
1901b: 197 (M, F). Type locality: Ellichpur [Central Provinces], India (NHMUK).
syn. punjabensis James
1911: 72 (A*; Myzomyia; as variety). In: James & Liston 1911. Type locality: Punjab, India (NHMUK).
syn. adenensis Christophers
1924c: 296 (A; as var.). Type locality: Dar al-A'mir, Aden Hinterland [Yemen] (NHMUK). References: Mattingly & Knight 1956: 95 (to subspecies); Harrison 1980: 52 (synonymy; lectotype designation).
CITED REFERENCES
Ahmed, T.U. (1987). Checklist of the mosquitoes of Bangladesh. Mosquito Systematics, 19(3), 187–200.
Baimai, V., Kijchalao, U., & Rattanarithikul, R. (1996). Metaphase karyotypes of Anopheles of Thailand and southeast Asia: V. The Myzomyia series, subgenus Cellia (Diptera: Culicidae). Journal of the American Mosquito Control Association, 12(1), 97–105
Becker, N., Petrić, D., Zgomba, M., Boase, C., Madon, M., Dahl, C., & Kaiser, A. (2010). Mosquitoes and their control (Second ed.). Berlin Heidelberg: Springer-Verlag.
Beidas, M.F., & Gillies, M.T. (1980). The egg of Anopheles (Cellia) culicifacies adenensis Christophers. Mosquito Systematics, 12(2), 172–174.
Christophers, S.R. (1924c). Some further varieties of Indian species of Anopheles with notes on the species A. pallidus Theobald and A. philippinensis Ludlow. Indian Journal of Medical Research, 12(2), 295–301.
Christophers, S.R. (1933). The fauna of British India, including Ceylon and Burma. Diptera.Vol. IV. Family Culicidae. Tribe Anophelini. London: Taylor and Francis.
Darsie, R.F., Jr., & Pradhan, S.P. (1990). The mosquitoes of Nepal: Their identification, distribution and biology. Mosquito Systematics, 22(2), 69–130.
Das, B. P. (1990). Morphological difference between sibling species of Anopheles culicifacies Giles in India: A preliminary report. Mosquito-Borne Diseases Bulletin, 7, 131–133.
De Meillon, B. (1947b). The Anophelini of the Ethiopian geographical region. Publications of the South African Institute for Medical Research, 10(49), 1–272.
Dev, V., & Sharma, V.P. (2013). The Dominant Mosquito Vectors of Human Malaria in India. In S. Manguin (Ed.), Anopheles mosquitoes - New insights into malaria vectors (pp. 239–271). Janeza Trdine 9, 51000 Rijeka, Croatia: InTech.
Evans, A.M. (1938). Mosquitoes of the Ethiopian Region. II. Anophelini adults and early stages. London: British Museum (Natural History).
Giles, G.M. (1901b). Description of four new species of Anopheles from India. Entomologist's Monthly Magazine, 37, 196–198.
Gillies, M.T., & de Meillon, B. (1968). The Anophelinae of Africa, south of the Sahara (Ethiopian zoogeographical region). Publications of the South African Institute for Medical Research, 54, 1–343.
Harrison, B.A. (1980). Medical entomology studies - XIII. The Myzomyia Series of Anopheles (Cellia) in Thailand, with emphasis on intra-interspecific variations (Diptera: Culicidae). Contributions of the American Entomological Institute, 17(4), 1–195.
Horsfall, W.R. (1955). Mosquitoes. Their bionomics and relation to disease. New York, NY: Hafner Publishing Company. (Reprinted 1972).
James, S.P., & Liston, W.G. (1911). A monograph of the anopheline mosquitoes of India, 2nd edition. Calcutta: Thacker, Spink and Co.
Kar, I., Subbarao, S.K., Eapen, A., Ravindran, J., Satyanarayana, T.S., Raghavendra, K., . . . Sharma, V. P. (1999). Evidence for a new malaria vector species, species E, within the Anopheles culicifacies complex (Diptera: Culicidae). Journal of Medical Entomology, 36(5), 595–600.
Kyalo, D., Amratia, P., Mundia, C.W., Mbogo, C.M., Coetzee, M., & Snow, R.W. (2017). A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Research, 2, 57.
Manonmani, A.M., Sadanandane, C., Sahu, S.S., Mathivanan, A., & Jambulingam, P. (2007). rDNA-ITS2-PCR assay for grouping the cryptic species of Anopheles culicifacies complex (Diptera: Culicidae). Acta Tropica, 104(1), 72–77.
Namgay,R., Drukpa, T., Wangdi, T., Pemo, D., Harbach, R.E., Somboon, P. (2018). A checklist of the Anopheles mosquito species (Diptera: Culicidae) in Bhutan. Acta Tropica 188 (2018) 206–212.
Nguyen Thuong Hien 1968. The genus of Anopheles in Vietnam. Saigon: Bureau of Entomology, National Malaria Program/ Republic of Vietnam. English translation by Military Entomology Information Service. 205pp.
Rattanarithikul, R., & Harrison, B.A. (1973). An illustrated key to the Anopheles larvae of Thailand. U S Army Medical Component, SEATO, Bangkok, Thailand.
Rattanarithikul, R., Harrison, B.A., Harbach, R.E., Panthusiri, P., & Coleman, R.E. (2006b). Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian Journal of Tropical Medicine and Public Health, 128(Supplement 2), 2.
Ross, E.S., & Roberts, H.R. (1943b). Mosquito atlas. Part II. Eighteen old world anophelines important to malaria. Contributions of the American Entomological Institute.
Sharma, A.K., Tyagi, V., Singh, S., Veer, V., Agrawal, O.P., & Sukumaran, D. (2014). Distribution of Anopheles culicifacies and detection of its sibling species E from Madhya Pradesh: Central India. Journal of Arthropod-Borne Deseases, 8(2), 186–196.
Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., ... Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4(1), 89.
Theobald, F.V. (1901a). A monograph of the Culicidae or mosquitoes (Vol. 1). London: British Museum (Natural History). [424pp]
Viswanathan, D.K., Rao, T.R., & Halgeri, A.V. (1955). Observations on some aspects of the nocturnal behavior of Anopheles culicifacies. Indian Journal of Malariology, 9, 371–384.
CITE THIS PAGE
Walter Reed Biosystematics Unit (Year). Anopheles culicifacies species page. Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/culicifacies, accessed on [date (e.g. 03 February 2020) when you last viewed the site].