AUSTRALASIA REGION
Etymology: Koli District, Guadalcanal
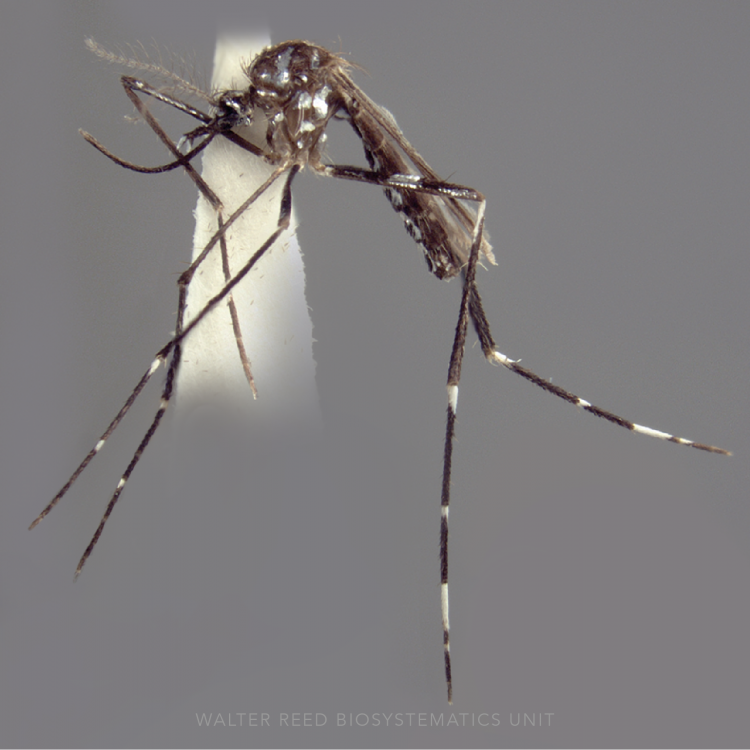
Anopheles koliensis has heavily spotted wings, a plethora of short white rings and spots on all its legs, and is unplaced within the Punctulatus Group (Neomyzomyia Series). Using the mtDNA cytochrome c oxidase II gene (COII) An. koliensis appears basal to all other taxa in the Punctulatus Group, using whole mtDNA genomes it is grouped with An. punctulatus and An. farauti 4, and is placed between An. farauti Laveran, and An. punctulatus Dönitz in studies using nuclear rDNA ITS2 sequences. Studies of An. koliensis in Papua New Guinea revealed three unique ITS2 PCR-RFLP genotypes for An. koliensis, suggesting the species may comprise additional cryptic taxa. The three variants were designated as M (Madang), W (Wosera), and MW (Madang-Wosera = classic type), however some interbreeding has been reported between M and MW forms.
Type locality: Koli Area, Guadalcanal, Solomon Islands
Type depository: U.S. National Museum, Washington, D.C., United States (USNM)
DIAGNOSTIC CHARACTERS (Click photos to view; mouse over and click large photo to zoom in.)
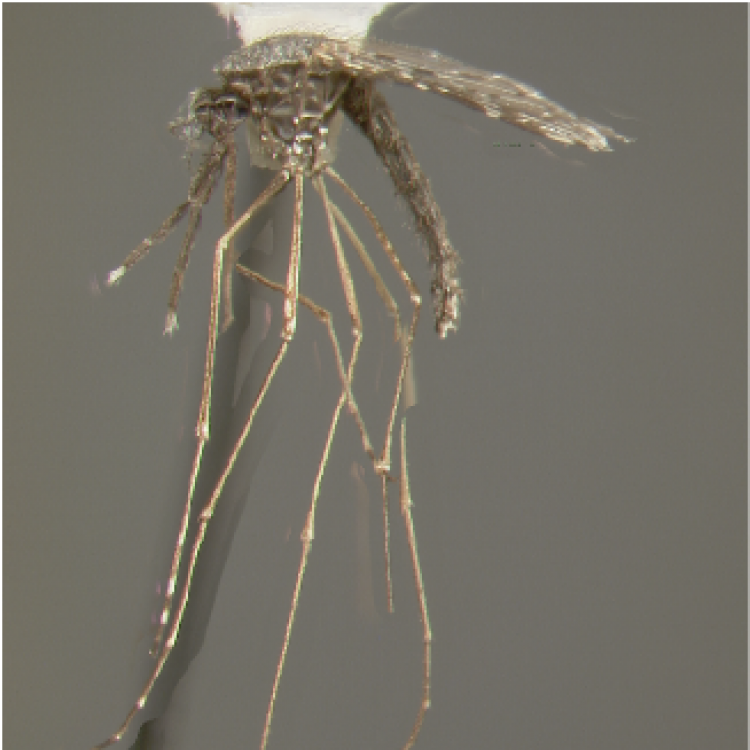
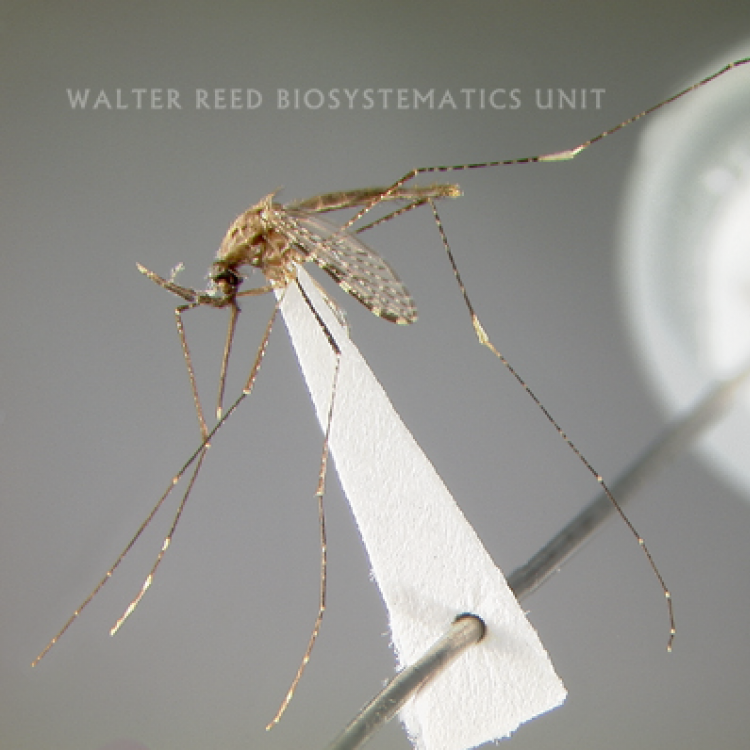
ADULT (illustrated): Head: Proboscis with pale scales on apical 0.33 to 0.50, in addition to apical pale band; MPlp3 with apical white portion divided by a black band; MPlp5 dark ventrally. Thorax: Scutum with scales on lateral areas. Mesepimeron without a central patch of flat pale scales; halter capitellum dark-scaled dorsally. Wing: Numerous dark spots; base of vein R with 1 or 2 black spots. Legs: Ta-I and Ta-II ringed or black and white spotted. Abdomen: Terga without dense scales.
LARVA (not illustrated): Head: Ventromentum relatively narrow and long. Thorax: Seta 1-M plumose; seta 1-P with stout expanded stem; setae 1–3-P on common tubercle; seta 9–12-P long, single. Abdominal segments: Seta 1-I not palmate, with slender or narrow hair-like branches. Terminal segments: Tergal plate of segment VIII small and those on IV,V approximately 2 x wider than deep; tergal accessory plates usually absent.
TAXONOMIC KEYS
None
![]()
WRBU – Anopheles - Neomyzomyia Series - Australasian Region - Larva
![]()
WRBU - Genera - Global - Adult
![]()
WRBU - Genera - Global - Larva
![]()
WRBU - Genera - Australasia - Adult
![]()
WRBU - Genera - Australasia - Larva
![]()
WRBU - Genera - Indomalaya - Adult
![]()
WRBU - Genera - Indomalaya - Larva
![]()
WRBU - Anopheles Subgenera and Series - Australasia - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Larva
Exemplar DNA sequences
An. koliensis COI: KU662130–306; partial mitochondrial genome JX219742–43; ITS2: KU139113-15.
BIONOMICS
Immatures
Typical immature habitats for An. koliensis are in peri-domestic sites. In West Papua thes include fresh water collections in drainage ditches, natural ground pools and agricultural plots of water spinach (Ipomea reptans). It is reported that short periods of drought lengthen the gonotrophic cycle of An. koliensis, leading to a reduction in biting activity and lowers local malaria cases. As rains fall and suitable immature habitats reappear, the gonotrophic cycle returns to normal and malaria transmission reignites.
Adults
Anopheles koliensis is found in lowland, inland environments, usually in close proximity to man. Only one specimen has ever been reported at elevations over 1,000 m. The typical form of An. koliensis is most often between 5–15 km from the sea, but can occasionally be detected closer to the coast. In Papua New Guinea, An. koliensis ITS genotype M was found along the Madang coast line, less than 5km from the sea, whereas An. koliensis W was found more than 30 km from the coast. Anopheles koliensis is endophilic, endophagous and highly anthropophilic, and one of the most effective malaria vectors in the Pacific Region. Insecticide-treated bednets and indoor residual spraying programs have been extremely successful against An. koliensis, almost driving it to extinction in the Solomon Islands and Buka and Bougainville islands in the Solomon archipelago.
DISTRIBUTION NOTES
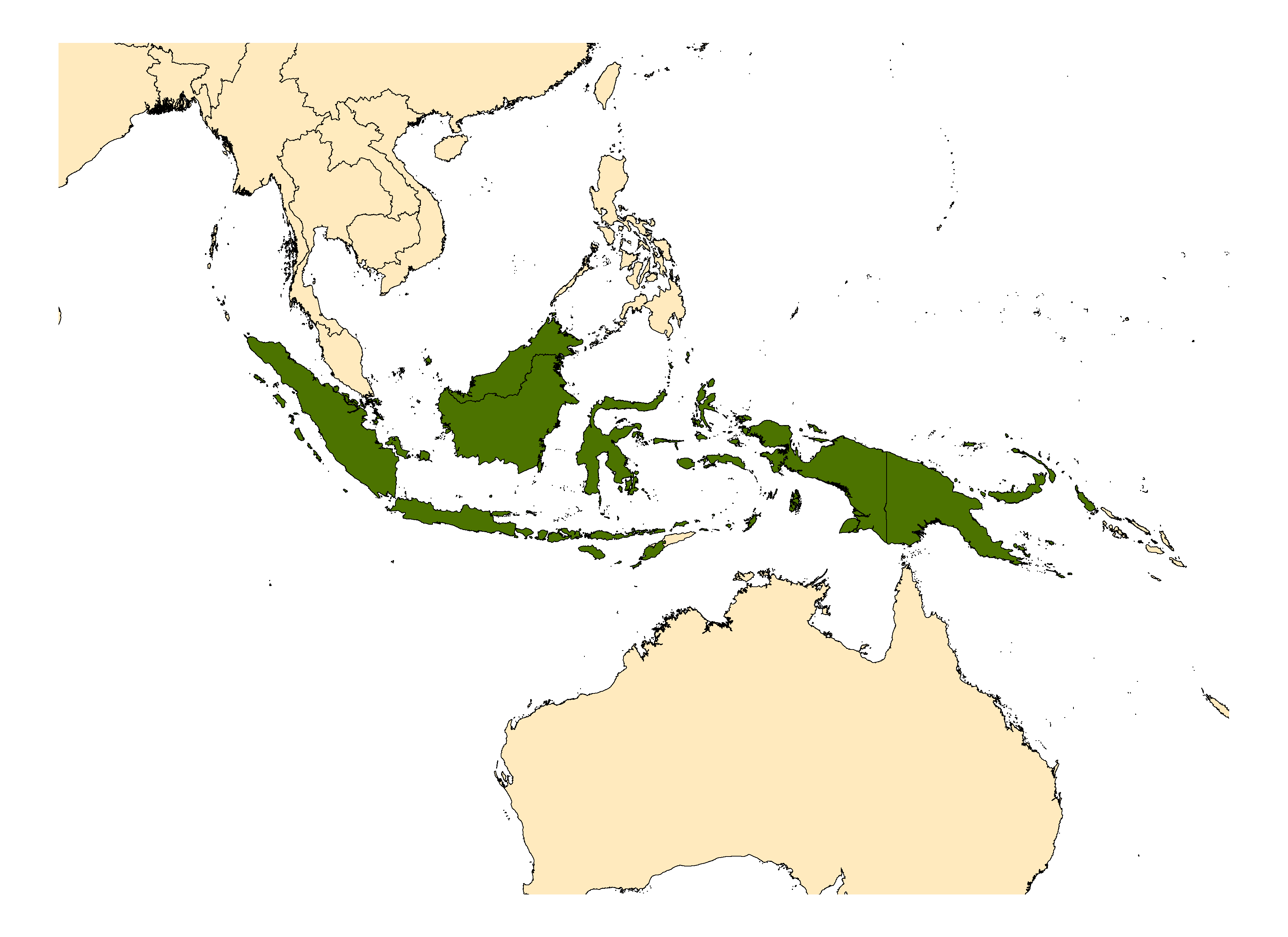
Indonesia (includes Kalimantan, Sulawesi), Papua New Guinea, Solomon Islands.
WRBU VECTOR HAZARD REPORTS
None; View other WRBU Vector Hazard Reports
Available GIS Models
IMPORTANT REFERENCES (full citations below)
Owen 1945: 236 (M, F*, L*)
Penn 1949b: 20 (P*)
Bonne-Wepster & Swellengrebel 1953: 349 (M, F*, L*)
Belkin 1962: 144 (M*, F*, P*, L*)
Bryan 1973: 64 (taxonomy)
Bryan 1974: 413 (taxonomy)
Lee et al. 1987b: 226 (taxonomy, literature review, distribution, bionomics)
Foley & Bryan 1993 (s.l.; molecular taxonomy)
Foley et al. 1993 (molecular taxonomy)
Benet et al. 2004 (molecular taxonomy, bionomics, distribution Papua New Guinea, as s.l.)
Cooper et al. 2009 (bionomics, biomedical importance)
Sinka et al. 2011: 89 (bionomics review, distribution, niche model)
Beebe et al. 2015 (phylogenetics, control, distribution; review)
CURRENT SYNONYMS
None
CURRENT SUBSPECIES
None
CITED REFERENCES
Beebe, N.W., Russell, T., Burkot, T.R., & Cooper, R.D. (2015). Anopheles punctulatus group: evolution, distribution, and control. Annual Review of Entomology, 60, 335–350.
Belkin, J.N. (1962). The mosquitoes of the South Pacific (Diptera, Culicidae) (Vols. 1 &2). Berkeley, California: University of California Press.
Benet, A., Mai, A., Bockarie, F., Lagog, M., Zimmerman, P., Alpers, M.P., Reeder, J.C. & M.J. Bockarie. (2004). Polymerase chain reaction diagnosis and the changing pattern of vector ecology and malaria transmission dynamics in Papua New Guinea. American Journal of Tropical Medicine and Hygiene, 71, 277–284.
Bonne-Wepster, J., & Swellengrebel, N.H. (1953). The anopheline mosquitoes of the Indo-Australian Region. Amsterdam: J. H. de Bussy.
Bryan, J.H. (1973). Studies on the Anopheles punctulatus complex I. Identfication by proboscis morphological criteria and by crossmating experiments. II. Hybridization of the member species. III. Mating behaviour of the F1 hybrid adults from crosses between Anopheles farauti no. 1 and Anopheles farauti no. 2. Transactions of the Royal Society of Tropical Medicine and Hygiene, 67(1), 64–91.
Bryan, J.H. (1974). Morphological studies on the Anopheles punctulatus Donitz complex. Transactions of the Royal Entomological Society of London, 125(4), 413–435.
Cooper, R.D., Waterson, D.G.E, Frances, S.P., Beebe, N.W., Pluess, B., Sweeeney, A.W. (2009). Malaria vectors of Papua New Guinea. International Journal for Parasitology 39, 1495–1501.
Foley, D.H., & Bryan, J.H. (1993). Electrophoretic keys to identify members of the Anopheles punctulatus complex of vector mosquitoes in Papua New Guinea. Medical and Veterinary Entomology, 7, 49–53.
Foley, D.H., Paru, R., Dagoro, H., & Bryan, J.H. (1993). Allozyme analysis reveals six species within the An. punctulatus complex of mosquitoes in Papua New Guinea. Medical and Veterinary Entomology, 7(1), 37–48.
Lee, D.J., Hicks, M.M., Griffiths, M., Debenham, M.L., Bryan, J.H., Russell, R.C., . . . Marks, E.N. (1987b). The Culicidae of the Australasian region. Volume 5. Commonwealth Department of Health, School of Public Health and Tropical Medicine Monograph Series, 2.
Owen, W.B. (1945). A new anopheline from the Solomon Islands with notes on its biology. Journal of Parasitology, 31, 236–240.
Penn, G.H. (1949b). The pupae of the mosquitoes of New Guinea. Pacific Science, 3, 3–85.
Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., ... Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4(1), 89.
Ye, Y.H., Carrasco, A.M., Frentia, F.D., Chenometh, S.F., Beebe, N.W., van den Hurk, A.F. …McGow, E.A. (2015). Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Neglected Tropical Diseases, 9(6), e0003894.
CITE THIS PAGE
Walter Reed Biosystematics Unit (Year). Anopheles koliensis species page. Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/koliensis, accessed on [date (e.g. 03 February 2020) when you last viewed the site].