INDO-ORIENTAL REGION
Etymology: Sunda Islands
Chromosomal and isoenzyme studies first exposed An. sundaicus as a species complex containing three distinct forms—the brackish–water forms A and C, and the freshwater inland form B. All three chromosomal forms were reported in sympatry at the costal locality of Asahan in North Sumatra, but only one genetic entity was later recovered at this site following the catastrophic tsunami of 2004. With the original type specimen misplaced, a neotype for An. sundaicus s.s. was described from Pandan Beach, Kuching, Sarawak (northern Borneo) and Form A, detected in coastal brackish-water populations from Trat Province in Thailand, was later described as An. epiroticus Linton & Harbach. Samples collected in South Tapanuli, Java (inland form B) were not genetically distinct from any other brackish water populations across SE Asia. Indonesian populations (collectively referred to An. sundaicus species D, not forms A, B or C) were genetically somewhat distinct from both An. sundaicus s.s. and An. epiroticus, and are reportedly most similar to Sri Lankan and Indian populations of An. sundaicus s.l.. Most recently, An. sundaicus species E, distinguished from all other taxa in the Sundaicus Complex on the basis of ITS2 sequences, was reported on the Car Nicobar Islands.
Type locality: Sunda Islands [Neotype: Pandan Beach near Lundu, Sarawak, Malaysia]
Type depository: Neotype in Sarawak Biodiversity Council, Kuching, Sarawak (SBC)
DIAGNOSTIC CHARACTERS (Click photos to view; mouse over and click large photo to zoom in.)
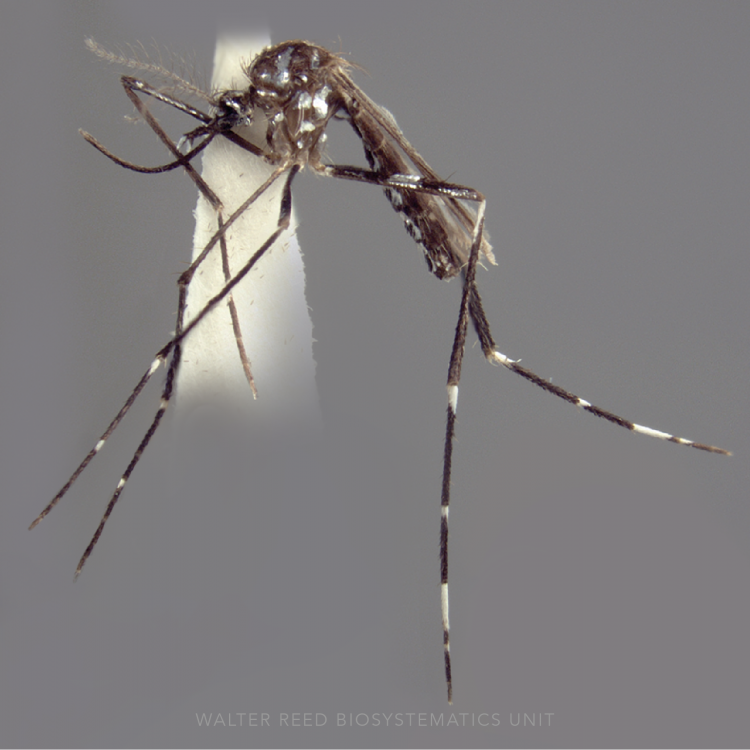
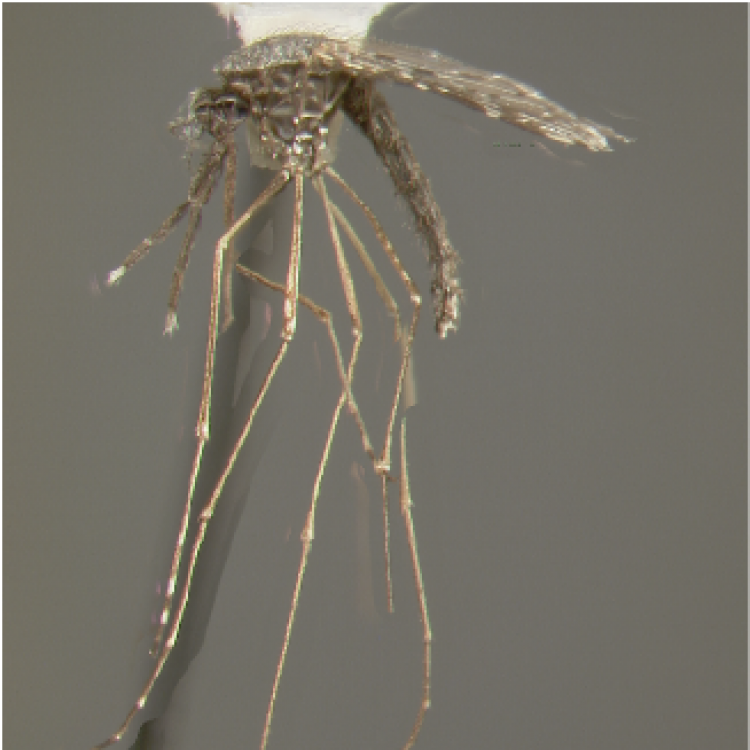
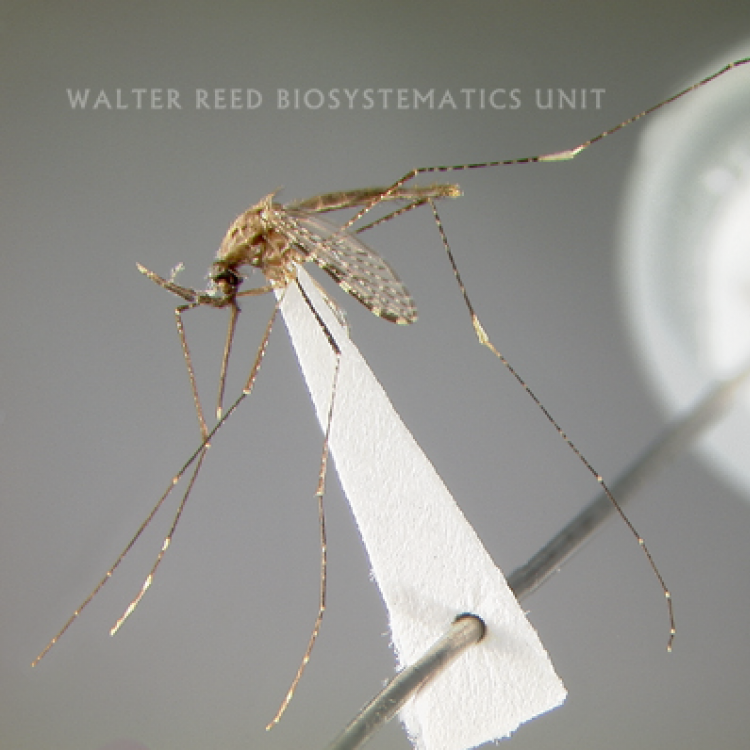
ADULT (illustrated): Head: Proboscis entirely dark-scaled; palpus with 3 pale rings, subapical dark band ≥0.5 x apical pale band and subapical pale band much narrower than apical band and equal to basal band. Thorax: Antepronotal scales absent; Upper proepisternal setae usually absent. Wing: Costa basal to humeral crossvein dark-scaled; pale fringe spot absent between cubital (CuA) and anal (1A) veins; vein 1A with 2 dark spots. Legs: Legs speckled with basal and apical pale bands on some tarsomeres; Ta-I with broad pale bands; apex of Ti-III and base of Ta-III1 without distinct contiguous white marking.
LARVA (not illustrated): Head: Seta 1-A single; seta 3-C ≥x 0.5 length of seta 2-C. Thorax: Seta 3-M not palmate; seta 4-M 3,4 branched from near base (not distinct in illustration); seta 1-P usually with ≥8 branches; setae 9,10,12-P,M single; setae 9,10-T branched or plumose. Abdominal segments: Seta 1-I palmate but less developed than on following segments; tergal plates on segments III-VI not very large, with concave posterior border not encompassing median posterior accessory tergal plate; distance between bases of abdominal palmate setae > width of anterior tergal plates; seta 6-IV–VI branched at base.
TAXONOMIC KEYS
Foote & Cook 1959
Lee et al. 1987b
Becker et al. 2010
![]()
WRBU – Anopheles – Pyretophorus Series – Indomalayan Region – Larva
![]()
WRBU – Anopheles – Pyretophorus Series – Oriental Region – Adult
![]()
WRBU – Anopheles – Pyretophorus Series – Oriental Region – Larva
![]()
WRBU - Genera - Global - Adult
![]()
WRBU - Genera - Global - Larva
![]()
WRBU - Genera - Indomalaya - Adult
![]()
WRBU - Genera - Indomalaya - Larva
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Larva
Exemplar DNA sequences
An. sundaicus s.s. COI: AY789709-26
BIONOMICS
Immatures
The neotype of An. sundaicus s.s. was collected from sunlit rock pools along a sandy shoreline in Pandan Beach, Sarawak, Malaysian Borneo. The habitats were shallow, sunlit, and contained submerged decaying leaves. The water was somewhat brackish, consisting mainly of rainwater with salt water spray from the nearby crashing waves. Anopheles epiroticus immatures are common in coastal regions of the mainland of Southeast Asia to India, predominating in brackish water fish/shrimp ponds and lagoons with filamentous green algae. In Indonesia, An. sundaicus D has been shown to tolerate a high range of salinity, ranging from 0—11%.
Adults
Female An. sundaicus s.s. were collected biting cattle and humans outdoors in villages along the coast of Sarawak, and at an inland site, Miri, Sarawak. In Miri, the density of An. sundaicus s.s. was phenomenally high, and the species would found in sympatry with An. barbirostris s.l..
DISTRIBUTION NOTES
Bangladesh, Cambodia, India, Indonesia, Malaysia (includes Sarawak), Myanmar, People's Republic of China, Philippines, Singapore, Taiwan, Thailand, Timor, Vietnam.
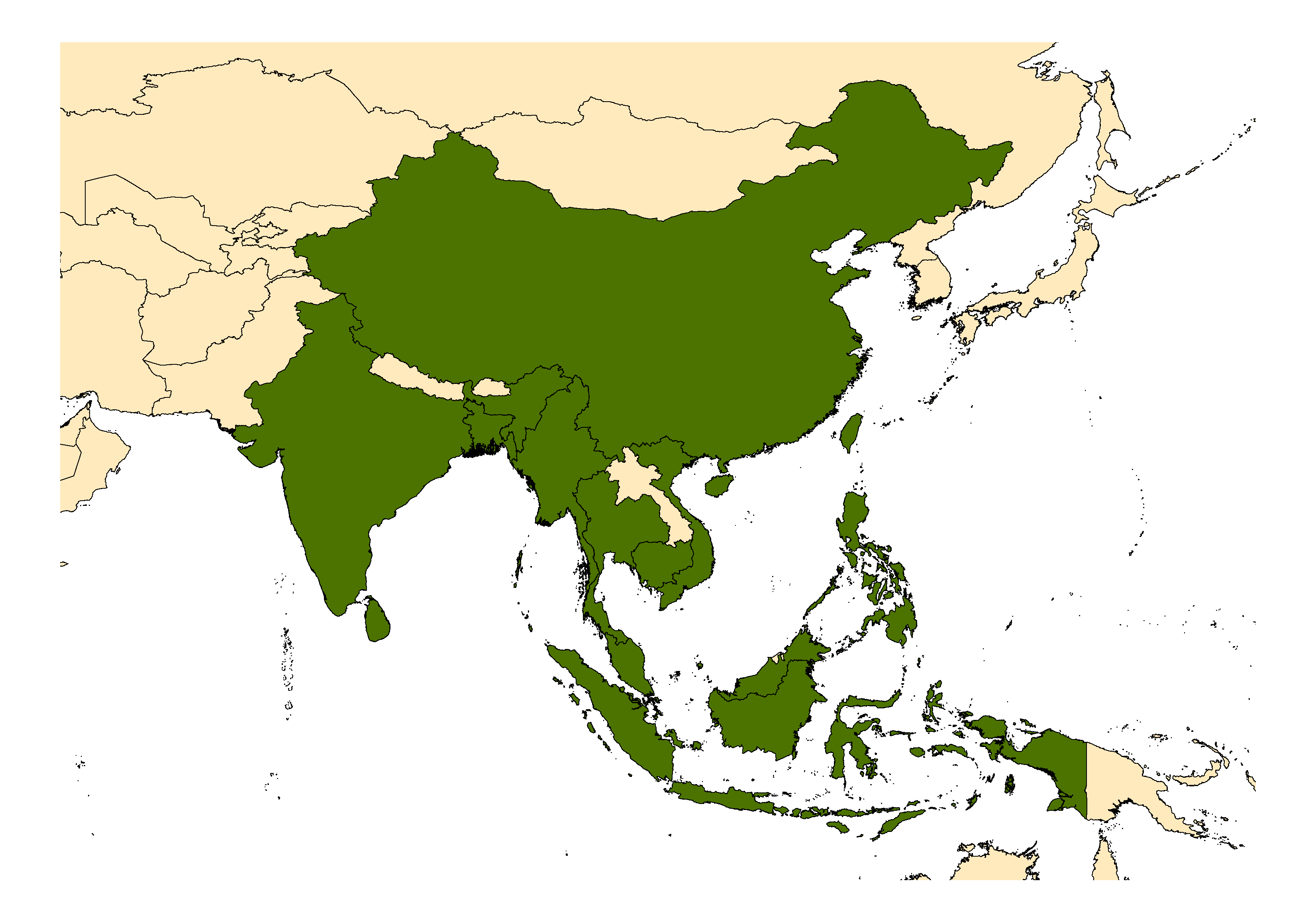
WRBU VECTOR HAZARD REPORTS
View other WRBU Vector Hazard Reports
Available GIS Models:
IMPORTANT REFERENCES (full citations below)
Rodenwaldt 1925: 185 (A*; Myzomyia ludlowi variety)
Christophers & Barraud 1931: 175 (E*)
Christophers 1933: 245 (M*, F*, L, E; to species)
Crawford 1938: 103 (P*)
Ross & Roberts 1943b: 23 (M*, F*, L*; taxonomy, distribution, bionomics)
Colless 1948: 111 (M*, F*, L*)
Bonne-Wepster & Swellengrebel 1953: 395 (M*, F*, L*)
Reid 1959: 101 (L)
Foote & Cook 1959 (key)
Reid 1968: 343 (M*, F*, P*, L*, E*; taxonomy)
Aslamkhan 1971b (distribution; Pakistan)
Lee et al. 1987b: 296 (F key, taxonomy, bionomics, distribution, review)
Sukowati & Baimai 1996 (chromosomes; s.l.)
Sukowati et al. 1999 (molecular taxonomy; s.l.)
Whelan & Hapgood 2000 (bionomics, distribution; East Timor)
Linton et al. 2001 (M*, F, P*, L*; molecular taxonomy, neotype designation)
Oo et al. 2004 (distribution; Myanmar)
Dusfour et al. 2004 (taxonomy, bionomics; s.l.)
Linton et al. 2005 (systematics; differentiation from epiroticus)
Dusfour et al. 2007 (taxonomy; s.l.)
Becker et al. 2010: 348 (F*, L*; key, taxonomy, distribution, bionomics)
Sinka et al. 2011: 89 (bionomics review, distribution, niche model; Sundaicus Complex)
Dev & Sharma 2013 (taxonomy, bionomics; Sundaicus Complex)
CURRENT SYNONYMS
None
CITED REFERENCES
Aslamkhan, M. (1971b). The mosquitoes of Pakistan I. A checklist. Mosquito Systematics, 3(4), 147–159.
Becker, N., Petrić, D., Zgomba, M., Boase, C., Madon, M., Dahl, C., & Kaiser, A. (2010). Mosquitoes and their control (Second ed.). Berlin Heidelberg: Springer-Verlag.
Bonne-Wepster, J., & Swellengrebel, N.H. (1953). The anopheline mosquitoes of the Indo-Australian Region. Amsterdam: J. H. de Bussy.
Christophers, S.R. (1933). The fauna of British India, including Ceylon and Burma. Diptera. Vol. IV. Family Culicidae. Tribe Anophelini. London: Taylor and Francis.
Christophers, S.R., & Barraud, P.J. (1931). The eggs of Indian Anopheles, with descriptions of the hitherto undescribed eggs of a number of species. Records of the Malaria Survey of India, 2(1), 161–192.
Colless, D.H. (1948). The anopheline mosquitoes of north-west Borneo. Proceedings of the Linnean Society of New South Wales, 73, 71–119.
Crawford, R. (1938). Some anopheline pupae of Malaya with a note on pupal structure. Singapore: Printed at the Government Printing Office, by W.T. Cherry, Government Printer.
Dev, V., & Sharma, V.P. (2013). The dominant mosquito vectors of human malaria in India. In S. Manguin (Ed.), Anopheles mosquitoes - New insights into malaria vectors (pp. 239–271). Janeza Trdine 9, 51000 Rijeka, Croatia: InTech.
Dusfour, I., Harbach, R.E., & Manguin, S. (2004). Bionomics and systematics of the Oriental Anopheles sundaicus complex in relation to malaria transmission and vector control. American Journal of Tropical Medicine and Hygiene, 71(4), 518–524.
Dusfour, I., Londeau, J., Harbach, R.E., Vythilingham, I., Baimai, V., Trung, H. D., . . . Manguin, S. (2007). Polymerase chain reaction identification of three members of the Anopheles sundaicus (Diptera: Culicidae) complex, malaria vectors in southeast Asia. Journal of Medical Entomology, 44(5), 723–731.
Dusfour, I., Michaux, J. R., Harbach, R.E., & Manguin, S. (2007). Speciation and phylogeography of the Southeast Asian Anopheles sundaicus complex. Infection Genetics and Evolution, 7(4), 484–493.
Foote, R.H., & Cook, D.R. (1959). Mosquitoes of medical importance. Agricultural Handbook, U.S. Department of Agriculture, 152, 1–158.
Lee, D.J., Hicks, M.M., Griffiths, M., Debenham, M.L., Bryan, J.H., Russell, R.C., . . . Marks, E.N. (1987b). The Culicidae of the Australasian region. Volume 5. Commonwealth Department of Health, School of Public Health and Tropical Medicine Monograph Series, 2.
Linton, Y.-M., Harbach, R.E., Seng, C.M., Anthony, T.G., & Matusop, A. (2001). Morphological and molecular identity of Anopheles (Cellia) sundaicus (Diptera: Culicidae), the nominotypical member of a malaria vector species complex in Southeast Asia. Systematic Entomology, 26(3), 357–366.
Linton, Y.-M., Dusfour, I., Howard, T. M., Ruiz L., F., Duc Manh, N., Ho Dinh, T., . . . Harbach, R.E. (2005). Anopheles (Cellia) epiroticus (Diptera: Culicidae), a new malaria vector species in the southeast Asian sundaicus complex. Bulletin of Entomological Research, 95(4), 329–339.
Oo, T.T., Storch, V., & Becker, N. (2004). Review of the Anopheles mosquitoes of Myanmar. Journal of Vector Ecology, 29(1), 21–40.
Reid, J.A. (1959). A note on the larvae of Anopheles subpictus and sundaicus. Mosquito News, 19, 101–102.
Reid, J.A. (1968). Anopheline mosquitoes of Malaya and Borneo. Studies from the Institute for Medical Research Malaysia, 31, 1–520.
Rodenwaldt, E. (1925). Entomologische notities. III. Geneeskundig Tijdschrift voor Nederlandsch Indie Batavia, 65, 173–201.
Ross, E.S., & Roberts, H.R. (1943b). Mosquito atlas. Part II. Eighteen old world anophelines important to malaria. Contributions of the American Entomological Institute.
Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., ... Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4(1), 89.
Sukowati, S., & Baimai, V. (1996). A standard cytogenetic map for Anopheles sundaicus (Diptera: Culicidae) and evidence for chromosomal differentiation in populations from Thailand and Indonesia. Genome, 39(1), 165–173.
Sukowati, S., Baimai, V., Harun, S., Dasuki, Y., Andris, H., & Efriwati, M. (1999). Isozyme evidence for three sibling species in the Anopheles sundaicus complex from Indonesia. Medical and Veterinary Entomology, 13, 408–414.
Whelan, P., & Hapgood, G. (2000). A mosquito survey of Dili, East Timor, and implications for disease control. Arbovirus Research in Australia, 8, 405–416.
CITE THIS PAGE
Walter Reed Biosystematics Unit (Year). Anopheles sundaicus species page. Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/sundaicus, accessed on [date (e.g. 03 February 2020) when you last viewed the site].