ORIENTAL REGION
Etymology: smallest (L); refers to being a very small species
Anopheles minimus is the nominotypical member of the Minimus Complex (Minimus Subgroup, Funestus Group), and has five valid synonyms—alboapicalis Theobald, christophersi Theobald, cohaesa Dönitz, formosaensis I Tsuzuki, and vincenti Laveran. The Minimus Complex comprises three undisputed taxa: Anopheles minimus s.s. (= form A), An. harrisoni Harbach & Manguin (formerly form C), and An. yaeyamensis Somboon & Harbach (formerly form E). There are no reliable morphological characters to distinguish members of the Minimus Group either as adults or immatures, thus molecular methods are widely applied. Following the neotype designation for An. minimus s.s., An. harrisoni was described based on unique sequences for the ITS2 and D3 loci of rDNA and the COI, COII and cyt b loci of mtDNA. Anopheles yaeyamaensis is discriminated from the other Minimus Group taxa by DNA sequences of the D3 and ITS2 regions of rDNA, and the mtDNA COII. The conspecificity of An. fluviatilis S (Fluvialitis Complex, Funestus Group) from India with Anopheles harrisoni from Thailand and Vietnam was proposed using 28S sequence comparisons, but the validity of this is under dispute. The status of two other putative entities—species D (chromosomal variant of A) and a D3 sequence variant (represented by a single specimen)—remain unresolved.
Type locality: Pokfulam, Hong Kong [People’s Republic of China]
Type depository: Location Unknown (LU)
DIAGNOSTIC CHARACTERS (Click photos to view; mouse over and click large photo to zoom in.)
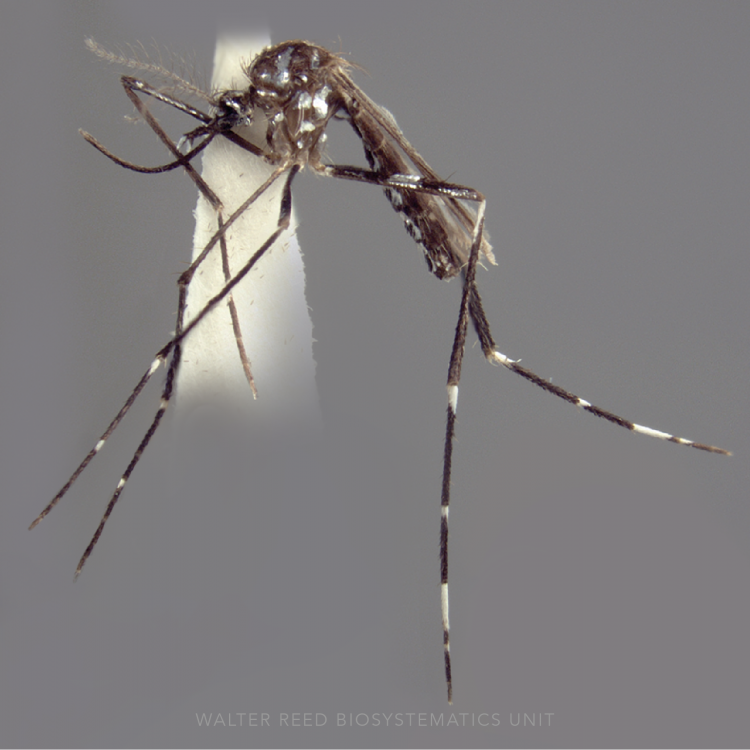
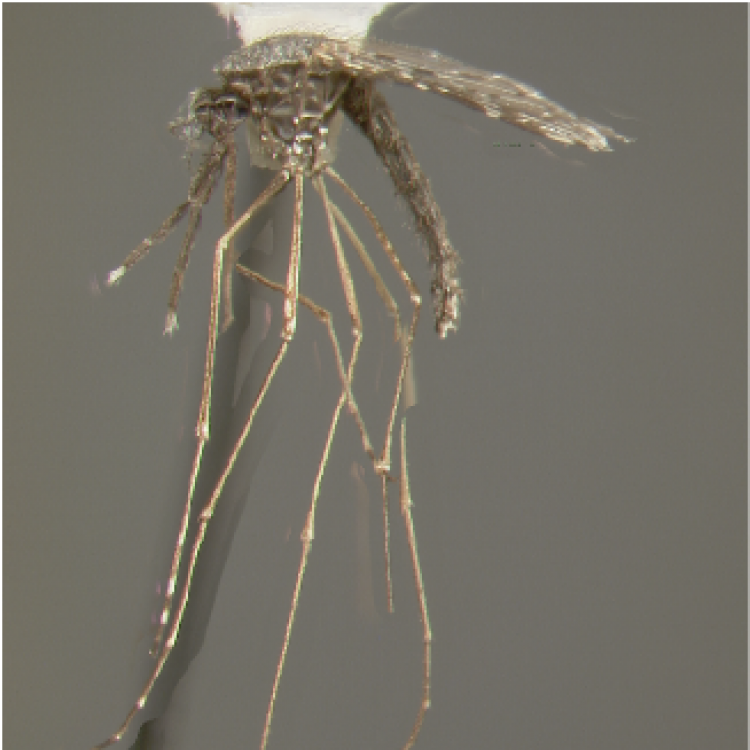
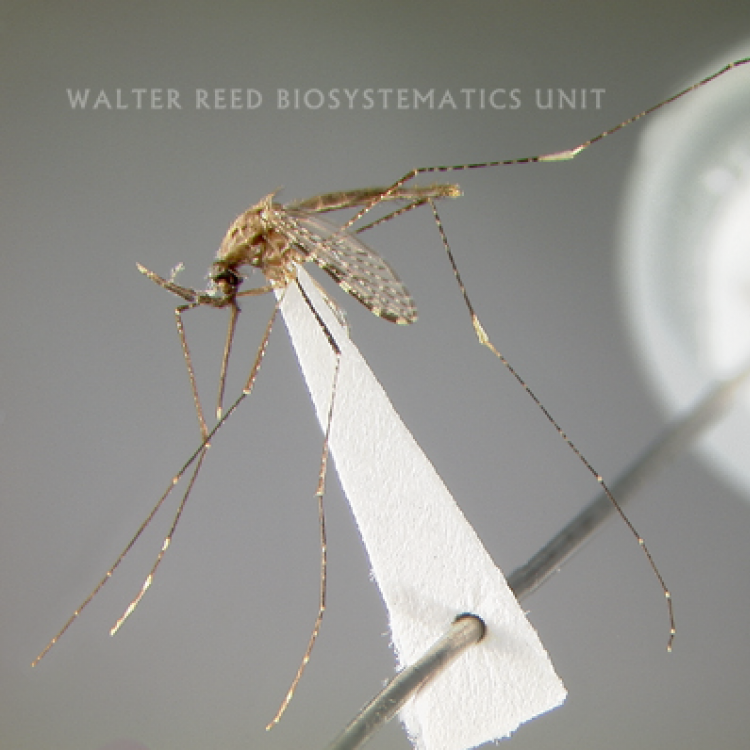
ADULT (illustrated): Head: Proboscis dark or with some pale scales ventrally; palpus with three pale bands, preapical dark band approximately equal to or shorter than apical pale band. Thorax: Scutum and scutellum with broad median pale pruinose area contrasting with darker lateral areas; scutum clothed with setae but not scales; no antepronotal scales; upper proepisternum with 1–4 brown to golden setae. Legs: Almost all dark, with indistinct pale marking on apices on Ti; Ta-I–III1–4 with faint dorsoapical pale spots and Ta-III5 dark-scaled. Wing: Remigium and base of vein R white-scaled; costa usually with presector pale spot but without humeral pale spot; base of R with only white or yellow-white scales; vein R2 dark except at base and apex; vein 1A with one long dark spot on distal half; hind margin of wing without pale fringe spot at vein 1A; vein M3+4 with two dark spots distal to mcu fork. Abdomen: Segments VII, VIII and ♀cercus without scales.
LARVA (not illustrated): Head: Seta 1-A small and single; seta 2-C simple, widely spaced. Thorax: Seta 4-M with sum of branches on both sides 8–11; setae 9,10-T with only one seta simple. Abdominal segments: Seta 0-IV–VI arising posterolateral to primary tergal plate, or at or on edge of plate; seta 0-IV–VII relatively large, 2–6-branched; anterior tergal plates on segments III–VII very large, 0.5 width of segment, enclosing median accessory tergal plate.
TAXONOMIC KEYS
Nguyen Thuong Hien 1968
Darsie & Pradhan 1990
Rattanarithikul et al. 2006b
Becker et al. 2010
![]()
WRBU – Anopheles - Myzomyia Series - Indomalayan Region - Larva
![]()
WRBU – Anopheles - Myzomyia Series - Oriental Region - Adult
![]()
WRBU – Anopheles - Myzomyia Series - Oriental Region - Larva
![]()
WRBU - Anopheles - Western Palearctic Region - Adult
![]()
WRBU - Genera - Global - Adult
![]()
WRBU - Genera - Global - Larva
![]()
WRBU - Genera - Indomalaya - Adult
![]()
WRBU - Genera - Indomalaya - Larva
![]()
WRBU - Genera - Oriental - Adult
![]()
WRBU - Genera - Oriental - Larva
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Larva
![]()
WRBU - Anopheles Subgenera and Series - Oriental - Adult
![]()
WRBU - Anopheles Subgenera and Series - Oriental - Larva
Exemplar DNA sequences
An. minimus A: 28S: AF114018, AF416782, AJ512720; ITS2: AB088379–80
An. harrisoni: 28S: DQ665847, AJ512719, AF425594, AF114017, AF437880 (as fluviatilis S, India ); ITS2: AF194506, DQ238493, AF416784 (as minimus C)
An. yaeyamaensis: 28S AB088039–40; ITS2: AB088376–77 (as minimus E)
BIONOMICS
Immatures
Little is known specifically about the exact biological preferences of members of the Minimus Group, as they can be identified only by DNA methods. Immature habitats of An. minimus s.s. and An. harrisoni in northern Vietnam and western Thailand (where the species occur in sympatry) are reportedly similar, with both species occupying clear waters in slow-flowing streams or canals, usually with grassy margins, however they have also been reported from rock pools, stream pools and in rice fields. Similar to invasive populations of An. stephensi Liston in Djibouti, and An. baimaii Sallum & Peyton in Myanmar, An. minimus s.s. in the suburbs of Hanoi, Vietnam have adapted to rearing in rain-filled water cisterns and wells. Anopheles yaeyamaensis immatures are found in clean streams, ground pools and springs. Increased water flow rates negatively impact the likelihood of finding An. minimus s.l. in any given habitat.
Adults
Anopheles minimus s.s. is opportunistic in its feeding preferences, feeding on any available large mammals. Anopheles harrisoni is predominantly zoophilic, but will feed on man. Anopheles harrisoni is exophagic and exophilic in northern Vietnam and western Thailand, and is believed to be a major malaria vector in southern China, where it will enter homes to feed. In Vietnam, the biting peak of An. harrisoni occurs before 2200h, after which An. minimus s.s. starts its peak biting activity. Adult An. harrisoni are most numerous in the dry seasons, before and after the rains. Anopheles yaeyamaensis adults are competent malaria vectors; populations significantly decrease in winter, when the active specimens appear to be larger and darker.
DISTRIBUTION NOTES
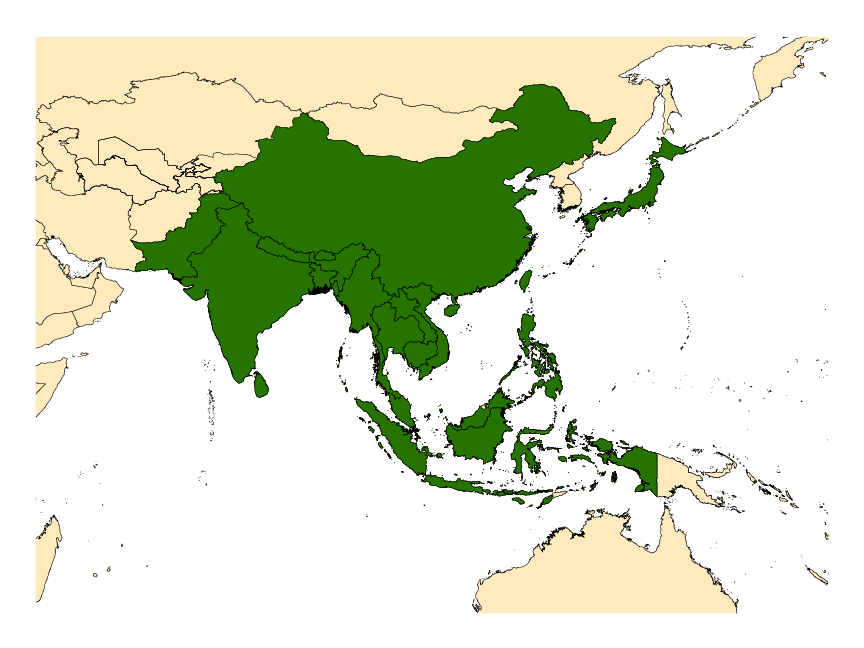
Bangladesh, Bhutan, Cambodia, China (including Hong Kong), India, Japan, Laos, Malaysia, Malta, Myanmar, Nepal, Pakistan, People’s Republic of China (includes Hong Kong), Sri Lanka, Taiwan, Thailand, Vietnam.
The recently clarified distribution of An. minimus s.l. includes Bangladesh, Cambodia, southern China, India, Japan, Laos, Malaysia, Myanmar, Nepal, Taiwan, Thailand, and Vietnam (see map). Molecularly confirmed distributions for the component taxa follow: Anopheles minimus s.s.—southern China (Including Hong Kong), Cambodia, Laos, Taiwan, Thailand and Vietnam; Anopheles harrisoni—southern China (Including Hong Kong), Laos, Thailand and Vietnam; and Anopheles yaeyamaensis—known only from the Yaeyama and Miyako Islands at the southern end of the Japanese Ryukyu Archipelago.
WRBU VECTOR HAZARD REPORTS
View other WRBU Vector Hazard Reports
Available GIS Models
IMPORTANT REFERENCES (full citations below)
Theobald 1901a: 186 (F*)
Christophers 1933: 209 (M*, F*, P, L, E)
Wu 1936: 269 (E*)
Ross & Roberts 1943b: 19 (M*, F*, L*; taxonomy, distribution, bionomics)
Bonne-Wepster & Swellengrebel 1953: 369 (M*, F*, L*)
Hara 1959: 110 (F*)
Nguyen Thuong Hien 1968 (F*, L*; keys, taxonomy, bionomics, Vietnam)
Reid 1968: 314 (M*, F*, P*, L*, E*; distribution)
Aslamkhan 1971b (distribution; Pakistan)
Tanaka et al. 1975c: 210 (bionomics, distribution)
Tanaka et al. 1979: 48 (M*, F*, L*)
Harrison 1980: 78 (M*, F*, P*, L*; distribution)
Darsie & Pradhan 1990 (F, L; tax., keys, bionomics, distribution; Nepal)
Green et al. 1990 (chromosomes; minimus sensu lato)
Sharpe et al 2000 (phylogeny; Minimus Group)
Chen et al. 2002 (molecular taxonomy)
Toma et al. 2003: 267 (distribution; Japan)
Oo et al. 2004 (distribution; Myanmar)
Garros et al. 2005b (taxonomy, bionomics)
Somboon et al (crossing experiments; minimus C, E)
Harbach et al. 2006 (M, F, P*, L*; neotype designation)
Rattanarithikul et al. 2006b (F*, L*; bionomics, distribution, keys)
Harbach et al. 2007 (taxonomy, genetics; minimus C = harrisoni)
Foley et al. 2008 (niche model, distribution)
Somboon et al. 2010 (systematics, taxonomy, bionomics, genetics; minimus E = yaeyamensis)
Becker et al. 2010: 346 (F*, L*; key, taxonomy, distribution, bionomics)
Chen et al. 2011 (molecular phylogenetics; sensu lato)
Sinka et al. 2011: 89 (bionomics review, distribution, niche model; Minimus Complex)
Dev & Sharma 2013 (taxonomy, bionomics; Minimus Complex.)
Namgay et al. 2018 (distribution, bionomics; Bhutan).
CURRENT SYNONYMS
syn. vincenti Laveran
1901c: 993 (F). Type locality: Van-Linh, Tonkin, Indochina [Hanoi Province, Vietnam] (PIP). References: Reid 1947: 88 (synonymy).
syn. christophersi Theobald
1902a: 378 (F*). Type locality: Duars [West Bengal], India (NHMUK). References: Harrison 1980: 83 (lectotype designation).
syn. formosaensis I Tsuzuki
1902b: 288 (M, F). Type locality: Formosa [Taiwan, ROC] (NHMUK; on slides). References: Harrison 1980: 83 (lectotype designation).
syn. cohaesa Dönitz
1903: 233 (aconitus variety, new name for formosaensis I Tsuzuki, Sept. 1902, not formosaensis II Tsuzuki, Feb. 1902).
syn. alboapicalis Theobald
1910c: 25 (F; christophersi variety). Type locality: Meenglas, Jalpaiguri & Duars, [West Bengal], India (NHMUK).
CURRENT SUBSPECIES
None
CITED REFERENCES
Aslamkhan, M. (1971b). The mosquitoes of Pakistan I. A checklist. Mosquito Systematics, 3(4), 147–159.
Becker, N., Petrić, D., Zgomba, M., Boase, C., Madon, M., Dahl, C., & Kaiser, A. (2010). Mosquitoes and their control (Second ed.). Berlin Heidelberg: Springer-Verlag.
Bonne-Wepster, J., & Swellengrebel, N.H. (1953). The anopheline mosquitoes of the Indo-Australian Region. Amsterdam: J. H. de Bussy.
Chen, B., Harbach, R.E., & Butlin, R.K. (2002). Molecular and morphological studies on the Anopheles minimus group of mosquitoes in southern China: taxonomic review, distribution and malaria vector status. Medical and Veterinary Entomology, 16, 253–265.
Chen, B., Pedro, P.M., Harbach, R.E., Somboon, P., Walton, C., & Butlin, R.K. (2011). Mitochondrial DNA variation in the malaria vector Anopheles minimus across China, Thailand and Vietnam: evolutionary hypothesis, population structure and population history. Heredity, 106(2), 241–252.
Christophers, S.R. (1933). The fauna of British India, including Ceylon and Burma. Diptera.Vol. IV. Family Culicidae. Tribe Anophelini. London: Taylor and Francis.
Darsie, R.F., Jr., & Pradhan, S.P. (1990). The mosquitoes of Nepal: Their identification, distribution and biology. Mosquito Systematics, 22(2), 69–130.
Dev, V., & Sharma, V.P. (2013). The Dominant Mosquito Vectors of Human Malaria in India. In S. Manguin (Ed.), Anopheles mosquitoes - New insights into malaria vectors (pp. 239–271). Janeza Trdine 9, 51000 Rijeka, Croatia: InTech.
Dönitz, W. (1903). Beitrage zur Kenntniss der Anopheles. II. Mittheilung. Zeitschrift fur Hygiene, 43, 215–238.
Foley, D.H., Weitzman, A.L., Miller, S.E., Faran, M.E., Rueda, L.M., & Wilkerson, R.C. (2008). The value of georeferenced collection records for predicting patterns of mosquito species richness and endemism in the Neotropics. Ecological Entomology, 33(1), 12–23.
Garros, C., Harbach, R.E., & Manguin, S. (2005b). Systematics and biogeographical implications of the phylogenetic relationships between members of the Funestus and Minimus Groups of Anopheles (Diptera: Culicidae). Journal of Medical Entomology, 42(1), 7–18.
Green, C.A., Cass, R.F., Munstermann, L.E., & Baimai, V. (1990). Population-genetic evidence for two species in Anopheles minimus in Thailand. Medical and Veterinary Entomology, 4, 25–34.
Hara, J. (1959). Taxonomical notes on the female terminalia of some anopheline mosquitoes of Japan and Formosa. Taxonomic and ecological studies on mosquitoes of Japan (Part 12). Japanese Journal of Experimental Medicine, 29, 107–119.
Harbach, R.E., Parkin, E., Chen, B., & Butlin, R.K. (2006). Anopheles (Cellia) minimus Theobald (Diptera: Culicidae): Neotype designation, characterization, and systematics. Proceedings of the Entomological Society of Washington, 108(1), 198–209.
Harrison, B.A. (1980). Medical entomology studies - XIII. The Myzomyia Series of Anopheles (Cellia) in Thailand, with emphasis on intra-interspecific variations (Diptera: Culicidae). Contributions of the American Entomological Institute, 17(4), 1–195.
Laveran, A. (1901c). Sur des culicides provenant du Haut-Tonkin.Comptes Rendus Société de Biologie, 53, 993–994.
Namgay,R., Drukpa, T., Wangdi, T., Pemo, D., Harbach, R. E., Somboon, P. (2018). A checklist of the Anopheles mosquito species (Diptera: Culicidae) in Bhutan. Acta Tropica 188 (2018) 206–212.
Nguyen Thuong Hien 1968. The genus of Anopheles in Vietnam. Saigon: Bureau of Entomology, National Malaria Program/ Republic of Vietnam. English translation by Military Entomology Information Service. 205pp.
Oo, T.T., Storch, V., & Becker, N. (2004). Review of the Anopheles mosquitoes of Myanmar. Journal of Vector Ecology, 29(1), 21–40.
Rattanarithikul, R., Harrison, B.A., Harbach, R.E., Panthusiri, P., & Coleman, R.E. (2006b). Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian Journal of Tropical Medicine and Public Health, 128(Supplement 2), 2.
Reid, J.A. (1947). Type specimens of Culicidae described by Laveran (Diptera: Culicidae). Proceedings of the Royal Entomological Society (B), 16, 86–91.
Reid, J.A. (1968). Anopheline mosquitoes of Malaya and Borneo. Studies from the Institute for Medical Research Malaysia, 31, 1–520.
Ross, E.S., & Roberts, H.R. (1943b). Mosquito atlas. Part II. Eighteen old world anophelines important to malaria. Contributions of the American Entomological Institute.
Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., ... Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4(1), 89.
Tanaka, K., Mizusawa, K., & Saugstad, E.S. (1979). A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and Ogasawara Islands) and Korea (Diptera: Culicidae). Contributions of the American Entomological Institute, 16, 1–987.
Tanaka, K., Saugstad, E.S., & Mizusawa, K. (1975c). Mosquitoes of the Ryukyu Archipelago (Diptera: Culicidae). Mosquito Systematics, 7(3), 207–233.
Theobald, F.V. (1901a). A monograph of the Culicidae or mosquitoes (Vol. 1). London: British Museum (Natural History). [424pp]
Theobald, F.V. (1902a). A short description of the Culicidae of India, with descriptions of new species of Anopheles. Proceedings of the Royal Entomological Society (B), 69, 367–394.
Theobald, F.V. (1910c). A monograph of the Culicidae of the World (Vol. V). London.
Toma, T., Miyagi, I., Murakami, H., Nerome, H., Yonamine, M., Higa, Y., & Tokuyama, Y. (2003). Distribution and seasonal prevalence of Anopheles minimus Theobald (Diptera: Culicidae) in the Yaeyama Island group (except Ishigaki Island), Ryukyu Archipelago, Japan, 1999–2000. Medical and Veterinary Zoology, 54(3), 267–274.
Tsuzuki, J. (1902b). Malaria und ihre Vermittler in Japan. Archiv fur Schiffs und Tropen-Hygiene, 6, 285–295.
Wu, S.C. (1936). Further notes on the mosquitoes of Hangchow, Chekiang, with description of one new species. Yearbook of the Bureau of Entomology Hangchow, 46–53.
CITE THIS PAGE
Walter Reed Biosystematics Unit (Year). Anopheles minimus species page. Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/minimus, accessed on [date (e.g. 03 February 2020) when you last viewed the site].