MIDDLE EASTERN, INDO-ORIENTAL & PALEARCTIC REGIONS
Etymology: not stated [of rivers (L); larval habitat streams]
Anopheles fluviatilis belongs to the Fluviatilis Complex, which are closely related to species in the Oriental Complex— sharing diagnostic characters of very large tergal abdominal plates in the larval stages. Anopheles fluviatilis is a species complex of three chromosomally identified forms (S, T & U), and these and two additional forms (V & X) have been recovered by genetic analysis. Form S (recently determined to be conspecific with An. minimus C) is common in India and Nepal. Form T, found in India, Iran, Nepal and Pakistan, exhibits three ITS2 haplotypes (T1, T2 and Y). Form U, closely related to form T, is found in Utter Pradesh, India and in southeastern Iran, and can reportedly hybridize. Forms V and X have recently been detected in Iran and India, respectively. Three synonyms of An. fluviatilis exist: listonii Liston, leptomeres Theobald and arabica, Christophers & Chand. The whereabouts of the type specimen of An. fluviatilis is unknown.
Type locality: Duars (Jalpaiguri District) [West Bengal], Nagpur [Central Provinces] & the Jeypore Hill Tracts, India
Type depository: Location Unknown (LU)
DIAGNOSTIC CHARACTERS (Click photos to view; mouse over and click large photo to zoom in.)
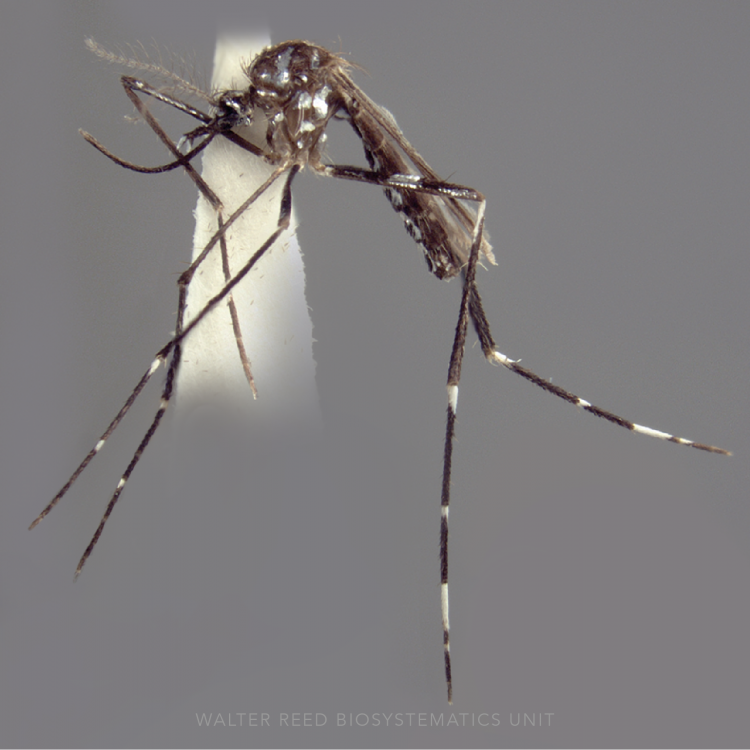
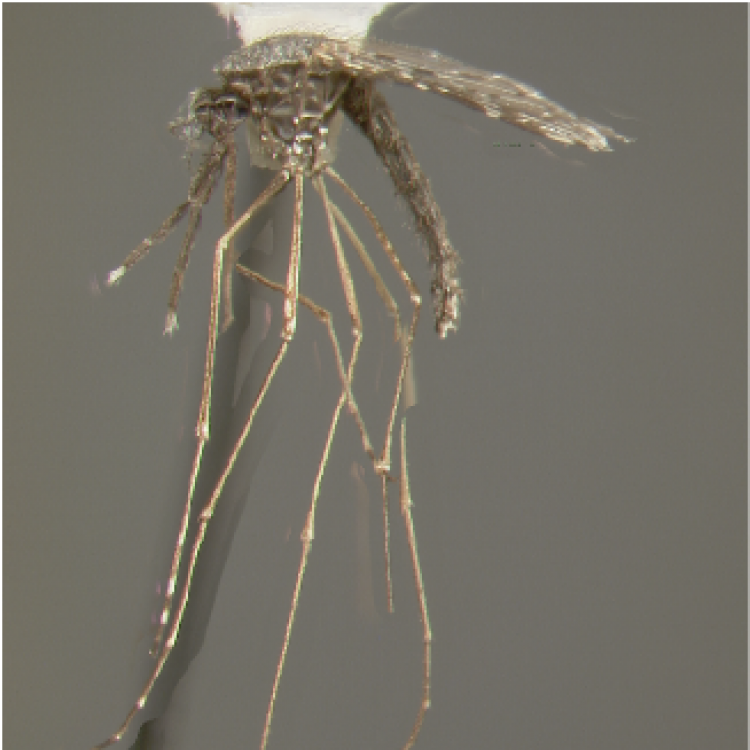
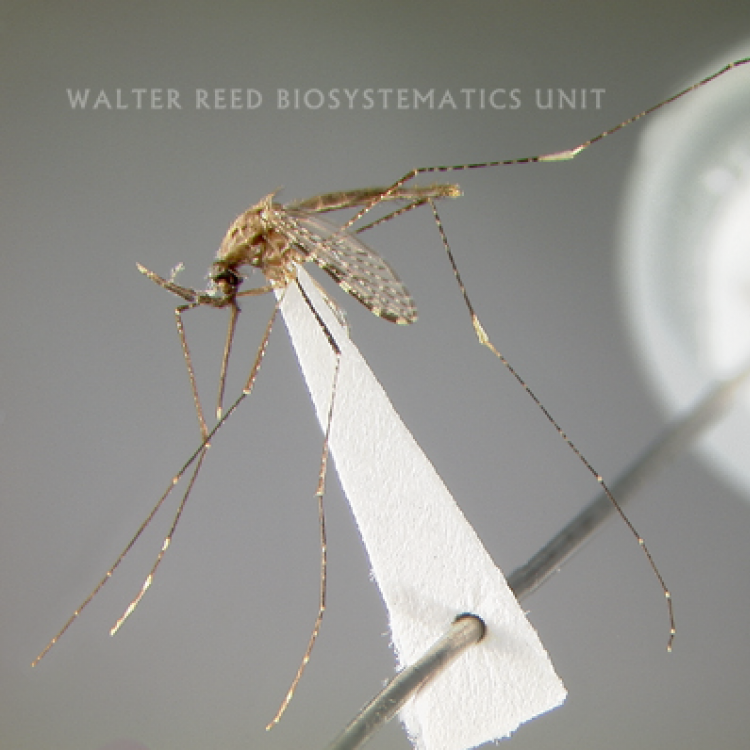
ADULT (illustrated): Head: Proboscis all dark; palpus with one broad and two narrower bands; MPlp4 with a narrow basal pale band, around half size of pale band on MPlp3; MPlp5 all pale; erect head scales broad, white on vertex and frontal tuft, and dark brown laterally and posteriorly. Thorax: Center of scutum mostly pale with narrow pale scales on median area; sides of scutum markedly darker than median area; upper proepisternal setae present; scutal fossa without scales. Wing: Costa entirely dark scaled in the basal third, with no pale spots basal to sector pale spot; radius with only white or yellowish scales just distal to humeral crossvein; vein R3 mostly dark; vein R4+5 mostly pale; wing fringe usually with at least four pale spots on posterior margin.
LARVA: Head: Seta 1-A small, single; seta 2-C simple, widely spaced. Thorax: Seta 4-M with sum of branches of both sides 8–11; one of setae 9,10-T unbranched. Abdominal segments: Anterior tergal plates on segments III–VII very large, >0.5 width of segment, enclosing accessory tergal plate; seta 0-IV–VI arising posterolateral to primary tergal plate, or at or on edge of plate; seta 0-IV–VII relatively large, 2–6-branched.
TAXONOMIC KEYS
Nguyen Thuong Hien 1968
Glick 1992
Becker et al. 2010
![]()
WRBU - Anopheles - Myzomyia Series - Oriental Region - Larva
![]()
WRBU - Anopheles - Western Palearctic Region - Adult
![]()
WRBU - Genera - Global - Adult
![]()
WRBU - Genera - Global - Larva
![]()
WRBU - Genera - Eastern Palearctic - Adult
![]()
WRBU - Genera - Eastern Palearctic - Larva
![]()
WRBU - Genera - Indomalaya - Adult
![]()
WRBU - Genera - Indomalaya - Larva
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Adult
![]()
WRBU - Anopheles Subgenera and Series - Indomalaya - Larva
Exemplar DNA sequences
An. fluviatilis COI: GQ906976–87
BIONOMICS
Immatures
Immature An. fluvialitis s.l. habitats include slow-flowing, heavily vegetated sites—stream pools, irrigation ditches, lake margins, ponds, swamp edges. In Yemen, immatures were collected at 7,300 ft, in pools within a ditch, heavily vegetated with filamentous green algae and stonewort rushes. Occasionally larvae have also been recovered from rice fields, and in water tanks.
Adults
In southern India, adult An. fluviatilis are associated with rocky foothills at low elevations (< 1,000 ft), but been reported as high as 6,000 ft in Kashmir and 7,500 ft in Muree (Pakistan). Adults appear to be strong flyers, with females captured over 2600 ft from their oviposition sites. Form S (= An. minimus C) starts host-seeking around 1.5 hours after dusk, and biting continues until midnight, and is regarded as a primary malaria vector in India. Form S (= An. minimus C) is strongly anthropophilic, feeding avidly on man, whereas forms T and U are exclusively or primarily zoophilic, preferring cattle.
DISTRIBUTION NOTES
Afghanistan, Bahrain, Bangladesh, Bhutan, Cambodia, India, Iran, Iraq, Israel, Kazakhstan, Laos, Myanmar, Nepal, Oman, Pakistan, People's Republic of China (includes Hong Kong), Saudi Arabia, Sri Lanka, Taiwan, Vietnam, Yemen.

WRBU VECTOR HAZARD REPORTS
View other WRBU Vector Hazard Reports
Available GIS Models
An_fluviatilis_s.l._Foley_1 Asia
IMPORTANT REFERENCES (full citations below)
James 1902: 31 (A*, L*)
Christophers 1933: 203 (M*, F*, P, L*, E)
Ross & Roberts 1943b: 17 (M*, F*, L*; taxonomy, distribution, bionomics)
Horsfall 1955: 214 (bionomics)
Bhombore et al. 1956 (bionomics)
Nguyen Thuong Hien 1968 (F*, L*; keys, taxonomy, bionomics, Vietnam)
Maffi 1971: 449 (L*; distribution)
Chowdaiah & Seetharam 1975: 495 (chromosomes*)
Harrison et al. 1991 (1990): 223 (distribution)
Glick 1992 (F*; key, Southwest Asia)
Nanda et al. 1996 (bionomics; fluviatilis S, T, U)
Oo et al. 2004 (distribution; Myanmar)
Singh et al. 2004 (molecular taxonomy)
Garros et al. 2005b (molecular taxonomy, morphology; Funestus & Minimus Groups)
Chen et al. 2006 (molecular taxonomy, systematics, distribution; fluviatilis X, Y)
Becker et al. 2010: 344 (F*, L*; key, taxonomy, distribution, bionomics)
Sinka et al. 2011: 89 (bionomics review, distribution, niche model; Fluviatilis Complex)
Dev & Sharma 2013 (taxonomy, bionomics; Fluviatilis Complex.)
Kumar et al. 2013 (molecular taxonomy)
Namgay et al. 2018 (distribution, bionomics; Bhutan)
CURRENT SYNONYMS
syn. listonii Liston
1901: 365 (A*; not Giles, 1901). Type locality: Ellichpur [Amaraoti District, Central Provinces], India (NHMUK). References: Christophers 1911a: 66 (E*).
syn. leptomeres Theobald
1903a: 38 (F; Myzomyia). Type locality: India (NHMUK).
syn. arabica Christophers & Chand
1915: 189 (M, F, L; funestus variety). Type locality: Muscat, [Oman] (NHMUK). References: Mattingly & Knight 1956: 92 (synonymy); Townsend 1990: 45 (type depository).
CITED REFERENCES
Becker, N., Petrić, D., Zgomba, M., Boase, C., Madon, M., Dahl, C., & Kaiser, A. (2010). Mosquitoes and their control (Second ed.). Berlin Heidelberg: Springer-Verlag.
Bhombore, S.R., Sitaraman, N.L., & Achuthan, C. (1956). Studies on the bionomics of Anopheles fluviatilis in Mysore State, India. II. Bionomics in Western Hill-tracts, Mysore State. Indian Journal of Malariology, 10(1), 23–33.
Chen, B., Butlin, R.K., Pedro, P.M., Wang, X.Z., Harbach, R.E (2006) Molecular variation, systematics and distribution of the Anopheles fluviatilis complex in southern Asia. Medical and Veterinary Entomology, 20(1), 33–43.
Chowdaiah, B.N., & Seetharam, P.L. (1975). Chromosome studies of oriental anophelines - IV. Polytene chromosomes of Anopheles fluviatilis. Mosquito News, 35(4), 495–500.
Christophers, S.R. (1911). [Notes on mosquitoes] II. A new anopheline, Paludism (Simla), 2, 64–68.
Christophers, S.R. (1933). The fauna of British India, including Ceylon and Burma. Diptera.Vol. IV. Family Culicidae. Tribe Anophelini. London: Taylor and Francis.
Christophers, S.R., & Chand, K. (1915). Notes on some anophelines from Arabia and Mesopotamia. Indian Journal of Medical Research, 3, 180–200.
Dev, V., & Sharma, V.P. (2013). The dominant mosquito vectors of human malaria in India. In S. Manguin (Ed.), Anopheles mosquitoes - New insights into malaria vectors (pp. 239–271). Janeza Trdine 9, 51000 Rijeka, Croatia: InTech.
Garros, C., Harbach, R.E., & Manguin, S. (2005b). Systematics and biogeographical implications of the phylogenetic relationships between members of the Funestus and Minimus Groups of Anopheles (Diptera: Culicidae). Journal of Medical Entomology, 42(1), 7–18.
Glick, J.I. (1992). Illustrated key to the female Anopheles of southwestern Asia and Egypt (Diptera: Culicidae). Mosquito Systematics, 24(2), 125–153.
Harrison, B.A., Rattanarithikul, R., Peyton, E.L., & Mongkolpanya, K. (1991). Taxonomic changes, revised occurrence records and notes on the Culicidae of Thailand and neighboring countries. Mosquito Systematics, 22 (1990)(3), 196–227.
Horsfall, W.R. (1955). Mosquitoes. Their bionomics and relation to disease. New York, NY: Hafner Publishing Company. (Reprinted 1972).
James, S.P. (1902) Malaria in India. Science of Medicine, Sanitary Department of India (New series), 2, 1–106.
Kumar, N.P., Krishnamoorthy, N., Sahu, S.S., Rajavel, A.R., Sabesan, S., & Jambulingam, P. (2013). DNA Barcodes indicate members of the Anopheles fluviatilis (Diptera: Culicidae) species complex to be conspecific in India. Molecular Ecology Resources, 13(3), 354–361.
Liston, W.G. (1901). A year's experience of the habits of Anopheles in Ellichpur. The description of the species of Anopheles found in Ellichpur during the year. Indian Medical Gazette, 36, 361–366, 441–443.
Maffi, M. (1971). On some larvae of the Myzomyia series collected in the Yemen. Parassitologia, 13, 449–458.
Mattingly, P.F., & Knight, K.L. (1956). The mosquitoes of Arabia. I. Bulletin of the British Museum (Natural History), 4(3), 89–141.
Nanda, N., Joshi, H., Subbarao, S.K., Yadav, R.S., Shukla, R.P., Dua, V.K., & Sharma, V.P. (1996) Anopheles fluviatilis complex: host feeding patterns of species S, T, and U. Journal of the American Mosquito Control Association, 29, 12(1), 147-149.
Namgay, R., Drukpa, T., Wangdi, T., Pemo, D., Harbach, R. E., & Somboon, P. (2018). A checklist of the Anopheles mosquito species (Diptera: Culicidae) in Bhutan. Acta Tropica, 188, 206–212.
Nguyen Thuong Hien (1968). The genus of Anopheles in Vietnam. Saigon: Bureau of Entomology, National Malaria Program/ Republic of Vietnam. English translation by Military Entomology Information Service. 205pp.
Oo, T.T., Storch, V., & Becker, N. (2004). Review of the Anopheles mosquitoes of Myanmar. Journal of Vector Ecology, 29(1), 21–40.
Ross, E.S., & Roberts, H.R. (1943b). Mosquito atlas. Part II. Eighteen old world anophelines important to malaria. The American Entomological Society, The Academy of Natural Sciences, Philadelphia, USA.
Singh, S.P., Raghavendra, K., Kumar, R., Nanda, N., & Subbarao, S.K. (2004). Morphotaxonomic studies to identify the members of the Anopheles subpictus Grassi (Diptera: Culicidae) species complex in riverine villages of District Sonepat, Haryana State, India. Journal of Communicable Diseases, 36(1), 35–40.
Sinka, M.E., Bangs, M.J., Manguin, S., Chareonviriyaphap, T., Patil, A.P., Temperley, W.H., ... Hay, S.I. (2011). The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites & Vectors, 4(1), 89.
Theobald, F.V. (1903a). A monograph of the Culicidae of the World (Vol. 3). London: British Museum (Natural History). 359pp
CITE THIS PAGE
Walter Reed Biosystematics Unit (Year). Anopheles fluviatilis species page Walter Reed Biosystematics Unit Website, http://wrbu.si.edu/vectorspecies/mosquitoes/fluviatilis, accessed on [date (e.g. 03 February 2020) when you last viewed the site].